Professional Guidance For Self
Mistletoe therapy can reduce the side effects of conventional cancer treatments, strengthen the immune system, and improve quality of life.
The first step
Mistletoe therapy is an individual therapy that requires medical supervision: Your doctor will advise you on the general suitability of mistletoe therapy, select the best type of mistletoe for your cancer, and familiarize you with the treatment. Then you will be able to continue the therapy independently at home.
The first treatment
Mistletoe products are usually injected under the skin two to three times a week, preferably at alternating sites on the abdomen or thigh.
For more information, watch our video:How-to video
How will my body react to mistletoe?
Mistletoe injections leave a few typical traces: temporary redness and minor swelling or itching at the injection site. You may also experience a slight increase in body temperature. These reactions are a completely normal, natural immune response to mistletoe. They show your immune system is working.
When should I contact a physician?
If redness is much larger than 5 cm in diameter. Or if you have a fever above 38 °C or any rare allergic reactions such as hives, facial swelling, or shortness of breath. In these cases, pause the mistletoe injections and contact your doctor to discuss adjusting the dosage or mistletoe type.
How long does mistletoe therapy last?
Mistletoe For Cancer Treatment
White berry mistletoe is a medicinal plant that has been in use for cancer treatment, in all stages of growth. Its use in Europe for sometime has been considered mainstream and is widely used, while in the U.S. many practitioners still regard it as complimentary . Not unlike a cancerous tumor, mistletoe is a semi-parasitic plant and possesses an advantage over other life forms as it can exist in an environment that does not have to compete for nutrients.
Discovered in 1917 by Dr. Ita Wegman, Dutch practitioner, created the first injectable mistletoe treatment, which today is considered a complimentary approach to oncololgy. The treatment can be employed before or after the following: surgery, chemotherapy, radiation, anti body or hormonal treatments. A doctor should administer initial treatment of mistletoe and educate the patient, after which the patient can inject their own or from the assistance of a caregiver. It is favored by those who know about its benefits since it is economical in cost and has few side effects.
Side Effects
Side effects can include fever, headache, chills and soreness and inflammation at injection sites. Most common treatments are given subcutaneously via injection less common are orally, into a vein , into the pleural cavity or directly into a tumor site.
Breast Cancer Treatment
Liver Cancer
From Luis Diaz, an associate professor of oncology in the Johns Hopkins School of Medicine on this case:
Studies of Mistletoe Extracts in Humans
Purpose Of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the use of mistletoe extracts in the treatment of people with cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Also Check: Does Breast Cancer Metastasis To Lung
You Can Do More For Yourself
Cancer is a very personal experience. For successful treatment, it is essential to consider your wishes and needs. For example, regaining vitality. Or reducing the uncomfortable side effects of standard, recognized therapies.
An increasing number of cancer patients trust mistletoe therapy, which frequently improves the tolerability of conventional cancer therapies and quality of life. Mistletoe therapy strengthens the immune system, reduces fatigue, normalizes moods, sleep patterns, and body temperature, among other effects.
You can do more for yourself. In every stage of your illness.
Mistletoe Extracts Patient Version

On This Page
- Mistletoe is a semiparasitic plant that grows on many types of trees, including apple, oak, pine, and elm .
- Mistletoe extracts are one of the most widely studied complementary and alternative medicinetherapies in people with cancer. In Europe, mistletoe extracts are among the most prescribeddrugs for patients with cancer .
- Mistletoe extracts are usually given by injection under the skin or, less often, into a vein, into the pleural cavity, or into a tumor .
- Few side effects have been reported from the use of mistletoe extracts .
- The U.S. Food and Drug Administration has not approved mistletoe extracts as a treatment for cancer or any other medical condition .
Also Check: Shoulder Pain And Breast Cancer
Questions And Answers About Mistletoe
Mistletoe is a semiparasitic plant that grows on many types of trees, including apple, oak, maple, elm, pine, and poplar. It has been used for hundreds of years to treat medical conditions such as epilepsy, asthma, hypertension, headaches, menopausalsymptoms, infertility, dermatitis, arthritis, and rheumatism.
Mistletoe extracts are one of the most widely studied complementary and alternative medicinetherapies for cancer. In Europe, mistletoe extracts are among the most prescribed therapies for cancer patients.
Mistletoe products vary, based on the following factors:
- Two randomized clinical trials that compared chemotherapy to mistletoe extract in non-small cell lung cancer patients reported no differences between the two groups in improved quality of life.
- A study done between 1978 and 1987 looked at the use of mistletoe extract in non-small cell lung cancer that could not be treated with surgery. Patients were randomly assigned to receive one of 3 treatments: a mistletoe extract injection an injection made from a sheep spleen said to stimulate the immune system and have antitumor effects a placebo injection of vitamin B. Results among the 3 groups were no different in survival or tumor response. It was noted that more patients in the mistletoe extract group than in the other groups reported an improved sense of well-being.
Melanoma
Reviews of combined clinical trials
Summary Discussion Conclusions And Recommendations
The HTA report on which this article is based contains a systematic literature review on efficacy and safety, costs and cost-effectiveness, patients and social aspects, and ethical evaluation.
Within the scope of the HTA report, no randomized controlled trials on the clinical efficacy of concomitant mistletoe therapy regarding overall survival in patients with breast cancer could be identified. One study , with a small sample size showed no difference in disease-free survival after five years between patients with and without concomitant mistletoe therapy.
There is evidence from three randomized controlled trials , , , that side effects of chemotherapy as measured by symptom scales are reduced, and health-related quality of life as measured by functional scales is increased. However, the effects are rather small to moderate. It is uncertain whether these effects could be due to systematic bias of the only subjectively measurable outcome measures due to insufficient blinding in the studies.
Known side effects of mistletoe therapy, such as mild and moderate local reactions, that were recorded in these three RCT , , , and four other non-randomized studies , , , , , , are common but of low magnitude. Possible interactions between anticancer drugs and mistletoe extracts, which could be due to immune stimulation, were not investigated in the included studies.
There are no sufficiently valid studies on the costs and cost-effectiveness of concomitant mistletoe therapy.
Recommended Reading: What Body Systems Are Affected By Breast Cancer
Icipant And Staff Interviews
Semi-structured interviews will be conducted with MAB study participants and BHOC staff to explore acceptability of the MAB therapy, therapy-related symptoms and administration of/participation in the trial. Interviewees will be selected via purposive sampling where enough exist otherwise, all participants who have indicated their consent to interview will be approached for interview, as well as relevant staff. These data will help plan the delivery and processes of the study therapy for the full trial and establish appropriate training needs. Pro-formas will be utilised in interviews, to include the following topics :
Interview 1 :
-
Understanding and expectations of MAB therapy
-
Awareness, interest in and use of complementary and/ or alternative therapies
-
Study processes including recruitment, administration of the MAB therapy and administration and completion of diaries and questionnaires
-
Local availability and perspectives on the role of CAM in cancer treatments in the NHS
Interview 2 :
-
Further exploration of the topics in interview 1 to identify any changes/clarification and overall views on the trial.
-
Participants understanding of the placebo effect will be investigated and their ideas on the MAB therapy treatment which they think they may have received.
To Learn More About Cam
National Center for Complementary and Integrative Health
The National Center for Complementary and Integrative Health at the National Institutes of Health facilitates research and evaluation of complementary and alternative practices, and provides information about a variety of approaches to health professionals and the public.
- NCCIH Clearinghouse
- Post Office Box 7923 Gaithersburg, MD 208987923
- Telephone: 1-888-644-6226
- TTY : 1-866-464-3615
- E-mail: info@nccih.nih.gov
You May Like: How Many Women Die Of Breast Cancer Every Year
Mistletoe For Breast Cancer
-
Mistletoe in early Breast Cancer : A pilot study for a randomised controlled trial
-
Research summary
Previous research has suggested that mistletoe treatment can improve quality of life of women with breast cancer, decrease fatigue and diminish the side effects of radiotherapy and chemotherapy. However, the evidence is not strong enough to make mistletoe part of routine treatment for breast cancer, not least because there are few comparisons between mistletoe and an identical looking non-active treatment . In this pilot study we will test whether a randomised controlled trial of mistletoe injections compared to placebo injections during chemotherapy or radiotherapy is feasible. We will find out whether women are willing to take part in such a trial, whether the mistletoe treatment or placebo is acceptable to them and whether they complete the questionnaires we are using to measure outcomes. At the end of the study we will ask participants whether they knew which treatment they were getting . The results of this pilot study will help us plan a large trial to test whether mistletoe does improve quality of life and reduce symptoms of women during breast cancer treatment.
Permission To Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as NCIs PDQ cancer information summary about breast cancer prevention states the risks in the following way: .
The best way to cite this PDQ summary is:
PDQ® Integrative, Alternative, and Complementary Therapies Editorial Board. PDQ Mistletoe Extracts. Bethesda, MD: National Cancer Institute. Updated < MM/DD/YYYY> . Available at: . Accessed < MM/DD/YYYY> .
Images in this summary are used with permission of the author, artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Don’t Miss: What Age Could You Get Breast Cancer
Icipant Diaries And Questionnaires
Participants will receive a diary card pack to record their study therapy and responses three times a week to correspond with the MAB study regime.
The MAB questionnaire pack comprises six questionnaires and will be administered at three time points during the trial: time point 0 or baseline following randomisation and before the start of chemotherapy regime time point one following the 3rd cycle of chemotherapy and time point two 4 weeks after the last standard treatment , on the day of the last study treatment. The questionnaires included are as follows:
European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire
EORTC QLQ-BR23 questionnaire
Functional Assessment of Cancer Therapy-Neutropenia scale
The CompleMentary and Alternative Beliefs Inventory
Questions To Ask Your Health Care Provider About Cam
When considering complementary and alternative therapies, patients should ask their health care provider the following questions:
- What side effects can be expected?
- What are the risks related to this therapy?
- What benefits can be expected from this therapy?
- Do the known benefits outweigh the risks?
- Will the therapy affect conventional treatment?
- Is this therapy part of a clinical trial?
- If so, who is the sponsor of the trial?
- Will the therapy be covered by health insurance?
Recommended Reading: How Big Are The Lumps In Breast Cancer
Inclusion And Exclusion Criteria
Potential participants will include adults 18 years or over with histologically verified early or locally advanced invasive breast cancer and with planned adjuvant chemotherapy, with or without radiotherapy and able to be randomised within 12 weeks of surgery. Patients who are to receive only radiotherapy will be excluded as this treatment is generally well tolerated and of short duration. They must be willing to self-administer or have a nominated person administer injections. Their Eastern Cooperative Oncology Group performance status must be 0 or 1, and they should have no active, uncontrolled infection. Female participants of childbearing age must be willing to adopt adequate contraceptive measures, and males must follow the chemotherapy guidance of the BHOC with regard to contraception. Patients will be excluded if they are receiving immunomodulatory therapy, receiving endocrine therapy as a stand-alone treatment, have previously had invasive breast cancer or bilateral breast cancer or have chronic viral infections such as hepatitis B and C and HIV known allergy to mistletoe, or be using/have had mistletoe within the last 5 years, acute inflammatory or pyrexial conditions, chronic granulomatous disease, active auto-immune diseases, or hyperthyroidism with tachycardia. Where appropriate, patients who are recommended to receive trastuzumab and/or endocrine therapy, as well as chemotherapy, are eligible.
Mistletoe Extract In Cancer: An Anthroposophic Remedy
May 1, 2005
Mistletoe Extract in Cancer: An Anthroposophic Remedy
By Melinda Ring, MD, Associate Program Director, Medicine Residency Program, and Coordinator, CAM Program, St. Joseph Hospital, Chicago, IL.
While many complementary practices, such as mind-body therapies, have little downside for cancer patients receiving treatment, the issue of safety with concomitant use of dietary supplements remains paramount. Not only can some supplements fail to help, but in certain cases they may lessen the efficacy of proven chemotherapy and radiotherapy regimens. Some patients forego conventional medical approaches to cancer treatment in favor of only using supplements, even when conventional approaches have been shown beneficial. Extreme caution should be taken, therefore, when approving the use of supplements in this setting, and then only when sufficient efficacy and safety data exist.
Proponents of mistletoe claim it stimulates the immune system, promotes cancer cell reversion to more differentiated forms, improves overall well-being, and may extend survival in certain cancers.5 Additionally, it is used for cancer prevention in high-risk patients, such as those with ulcerative colitis, cervical dysplasia, papillomatosis of the bladder, and intestinal polyposis.
History
Laboratory Evidence/Active Constituents
Clinical Evidence
Some of the more rigorous studies explored in these reviews are summarized below.
You May Like: Can Cancer Spread To The Breast
Mistletoe Treatment In Cancer Patients
Preparations from the European mistletoe are among the most prescribed drugs in cancer patients in several European countries. Proponents claim that mistletoe extracts stimulate the immune system, improve survival, enhance quality of life and reduce adverse effects of chemo- and radiotherapy in cancer patients. The review found that there was not enough evidence to reach clear conclusions about the effects on any of these outcomes and it is therefore not clear to what extent the application of mistletoe extracts translates into improved symptom control, enhanced tumour response or prolonged survival. Adverse effects of mistletoe extracts were reported, but appeared to be dose-dependent and primarily confined to reactions at injection site and mild, transient flu-like symptoms. In the absence of good quality, independent trials, decisions about whether mistletoe extracts are likely to be beneficial for a particular problem should rely on expert judgement and practical considerations.
Mistletoe extracts are commonly used in cancer patients. It is claimed that they improve survival and quality of life in cancer patients.
To determine the effectiveness, tolerability and safety of mistletoe extracts given either as monotherapy or adjunct therapy for patients with cancer.
Data on side effects indicated that, depending on the dose, mistletoe extracts were usually well tolerated and had few side effects.
Not All Mistletoe Is The Same
Only European white-berry mistletoe is suitable for medicinal use. Depending on how it attaches to the host, its ingredient composition varies. There are three types of white-berry mistletoe, which grow on different host trees. Your physician will choose the correct variety depending on tumor type and individual considerations.
Fir mistletoe
Don’t Miss: Does Dairy Cause Breast Cancer
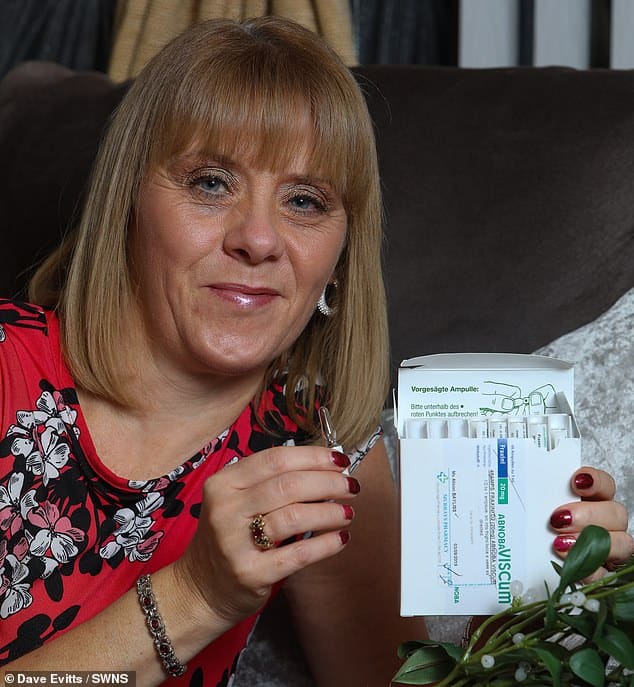
Are Mistletoe Extract Injections The Next Big Thing In Cancer Therapy
In September 2008, Ivelisse Page, a 37-year-old mother of four, was diagnosed with colon cancer. Several weeks later, she had 15 inches of her colon and 28 lymph nodes removed. But in December of that same year, Page’s doctor, Luis Diaz, an associate professor of oncology in the Johns Hopkins School of Medicine, had to deliver the devastating news that the cancer had spread to her liver. He told her that she had just an 8 percent chance of surviving for more than two years.
Page had more surgery to remove 20 percent of her liver, but instead of undergoing conventional chemotherapy, she pondered the suggestion of another of her doctors, Peter Hinderberger of Baltimore’s Ruscombe Mansion Community Health Center. A specialist in using complementary therapies, Hinderberger had seen positive effects from injections of mistletoe extract. The liquid, derived from the poisonous, semiparasitic mistletoe plant, has been a popular natural remedy in treating cancer in Europe for years, but Hinderberger is one of the few physicians nationwide who regularly use the therapy.
The next time the doctor saw his patient, he was amazed. “The one thing I noticed was that as soon as she went on it, she started feeling better,” he recalls. “That’s a universal feature I’ve seen in all patients who get mistletoe. Their improves they have more energy.”
Cancer research
Related coverage of the efforts and new insights in the fight against cancer