Are There Any Side Effects Of Treatment And How Can I Manage Them
Treatment side effects will depend on the type of treatment you undergo. In general, patients can tolerate monoclonal antibodies used to target HER2-positive receptors well.
Some potential side effects include fatigue, joint pain, headache, and insomnia. A majority of these side effects are minor in severity.
Rarely, monoclonal antibodies used to treat HER2-positive breast cancer can cause a weakening of the heart muscles. Your oncology team will discuss this risk with you and monitor you closely for any signs of this rare complication.
Who May Not Be Able To Have Herceptin
Herceptin should not be used to treat people with breast, oesophageal or stomach cancer that is not HER2 positive.
It may also not be suitable if:
- you have a pre-existing heart condition, such as heart failure, severe angina or a problem with your heart valves
- you have poorly controlled high blood pressure
- you’re pregnant
- you’re breastfeeding
Avoid becoming pregnant while taking herceptin and for at least 7 months after treatment stops, as it could harm your developing baby.
Also avoid breastfeeding until at least 7 months after treatment stops, as the medicine can enter breast milk and may be harmful for babies.
Which Unmet Needs In Patients With Her2
A very important study is the phase 3 DESTINY-Breast04 trial , which showed there was benefit in the HER2-low subgroup. Right now, there is no treatment for the HER2-low subgroup. Its not even characterized as a separate subgroup. However, with the data that we are seeing with trastuzumab deruxtecan, specifically in DESTINY-Breast04, we are going to focus on this subgroup, and it will be interesting to see how trastuzumab deruxtecanor any of the other antiHER2-targeted therapies will show efficacy in this space.
The DESTINY-Breast04 study did meet its primary end point, which was PFS, secondary end points, such as OS in the hormone receptorpositive subgroup of patients.
Some interesting combinations would be CDK4/6 inhibitors with trastuzumab deruxtecan. We will have to do a phase 1 study to see how the tolerability of this combination is going to be with hormonal therapy. There are a lot more interesting trials that will be coming out, looking at different combinations in this new subtype of breast cancer, the HER2-low tumors.
Recommended Reading: Can Sleeping On Your Breast Cause Cancer
Taking Care Of Yourself
Having breast cancer can be overwhelming. Remember, though: You’re in control of your treatment decisions and how you live your life.
ishonestNo.172 – Pre-Sun Exposure
These tips can help you stay healthy while you get treatment:
- Get the support you need. This could be information about breast cancer, talking with someone, or help with daily tasks. It can all make a huge difference in how you feel. Listen to your body. Exercise can help you feel better, but only when you’re up for it.
- Stay nourished. If you don’t have much of an appetite, eat smaller meals every few hours rather than three big meals.
Different Drugs Same Target

Breast cancers that are HER2-positive tend to be aggressive, with the excess HER2 protein on tumor cells fueling the cancers growth. In the late 1990s, trastuzumab was among the first targeted cancer therapies to be approved by FDA, after trials showed it could improve survival in women with metastatic HER2-positive breast cancer.
Over time, other HER2-targeted therapies emerged, some with alternative mechanisms for disrupting HER2 activity in cancer cells. Drugs like trastuzumab and pertuzumab are monoclonal antibodies that bind to the HER2 protein above the cancer cells surface, preventing it from acting or enlisting the immune system to help destroy cells that produce it.
Tucatinib, on the other hand, is a member of a class of drugs known as tyrosine kinase inhibitors . These drugs work by binding to the part of the HER2 protein that is inside the cell and preventing it from sending signals that promote cell growth. Other HER2-targeted TKIs include neratinib and lapatinib .
Some TKIs have multiple targets. But, compared with other HER2-targeted drugs, tucatinib appears to be relatively selective for HER2that is, its less likely to bind to related proteins, explained Stanley Lipkowitz, M.D., Ph.D., chief of the Womens Malignancies Branch in NCIs Center for Cancer Research. That selectivity limits the risk of side effects seen with other HER2-targeted TKIs that inhibit other targets, Dr. Lipkowitz said.
Also Check: Chances Of Surviving Stage 4 Breast Cancer
Exploring Why Some Cancers Grow And Spread And Others Dont
For years, doctors and researchers have noted that not all cancers are alike. Some patients tumors grow quite slowly and never spread beyond the site where they first formed. But for other patients, their tumors grow rapidly and spread like wildfire.
In the early 1980s, after the discovery that a mutated gene called HER2 could stimulate excessive cell growth and division, many scientists wondered if certain genes might make cancers grow and spread rapidly. Researchers around the world began searching for genes that spur cancer growth.
Heart Problems Linked To Trastuzumab
Trastuzumab use is linked to congestive heart failure, a serious heart condition.
In clinical trials, about 2-3 percent of those treated with chemotherapy plus trastuzumab had heart failure, compared to fewer than 1 percent of those treated with chemotherapy alone .
This risk of heart problems is higher with chemotherapy regimens that include an anthracycline drug compared to regimens without an anthracycline drug .
The risk of heart problems may also be higher for women over 60 and for those who already have heart problems .
For most people who develop a heart problem while taking trastuzumab, the condition improves after stopping trastuzumab. For a few, it may be permanent.
Avoiding heart problems
Your heart will be checked before and during treatment with trastuzumab. This is to help make sure there are no problems, or if there are problems, theyre caught early.
Adopting a lifestyle that includes a healthy diet, regular exercise and for those who smoke, quitting smoking are good ways to help avoid heart problems related to treatment.
To learn more about trastuzumab, visit the National Institutes of Healths Medline Plus website.
Recommended Reading: What Is The Blood Test For Breast Cancer
Treatment In The First
The CLEOPATRA trial examined the addition of pertuzumab to the doublet trastuzumab + docetaxel as first-line treatment in patients with HER2-positive metastatic breast cancer . Results demonstrated an improvement in median progression-free survival and an even greater improvement in overall survival with the addition of pertuzumab to the doublet in the first-line setting . As a result of the CLEOPATRA trial, pertuzumab + trastuzumab + docetaxel was approved as the standard of care for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease .
In a more recent analysis after a median follow-up of 99.9 months, the 8-year landmark OS rates were 37% with the addition of pertuzumab and 23% in the doublet group, demonstrating a long-term survival benefit after first-line treatment with the addition of pertuzumab . Moreover, the 8-year landmark PFS rates were 16% with the addition of pertuzumab and 10% in the doublet group, suggesting that a subgroup of patients might be cured after first-line treatment. Patients who present with de novo metastatic disease, not previously exposed to HER2-targeting agents, are more likely to experience long-term disease control, particularly with oligometastatic disease . PFS and OS estimates are strikingly good for these patients if they can achieve a no-evidence-of-disease status.
Moffitt Is Addressing That Need
In clinical trials led by the investigators in our Don & Erika Wallace Comprehensive Breast Program, Moffitt is studying new targeted drugs that show promise for treating patients with advanced HER2-positive breast cancer that is no longer responding to standard therapies. Our goal is to identify drugs that can serve as a new line of attack against HER2 proteins that have become desensitized to the effects of other drugs. To date, studies of the clinical effectiveness of these drugs confirm that we are making significant progress toward this goal.
If you have questions or would like to explore our HER2-positive breast cancer clinical trials that are currently accepting new patients, you can request an appointment with an oncologist in Moffitts breast cancer program by calling or completing our new patient registration form online. We can provide individualized guidance to help you find the optimal treatment options.
You May Like: How Do You Know If You Have Breast Cancer
Comparing Two Targeted Treatments
In about 15%20% of people with breast cancer, tumors overproduce HER2, with the excess HER2 on tumor cells driving the cancers growth. Such HER2-positive tumors tend to grow faster and are more likely to spread elsewhere in the body, or metastasize, than those that do not overproduce HER2.
Trastuzumab and other drugs called monoclonal antibodies that target HER2 have been quite successful as treatments for HER2-positive breast cancer. As a result, researchers have been developing new drugs based on these antibodies.
Both T-DM1 and T-DXd, which are given by infusion into a vein, are drugs known as antibodydrug conjugates . Such drugs consist of a monoclonal antibody, in this case trastuzumab, chemically linked to a cell-killing chemotherapy drug.
The trastuzumab component of both T-DM1 and T-DXd acts as a homing device that helps the drug deliver its chemotherapy payload directly to tumor cells that overproduce HER2. The ADC is then ferried inside the cell, where the attached chemotherapy drug is released.
The new study, a large international clinical trial called DESTINY-Breast03, is the first to directly compare T-DXd with another treatment in people with breast cancer. The trial was funded by Daiichi Sankyo, Inc., and AstraZeneca, the developers of T-DXd.
We were waiting for T-DXd to be compared to another treatment in a large clinical trial like , Dr. Zimmer continued.
Trastuzumab Targets Breast Cancer In Clinical Trials
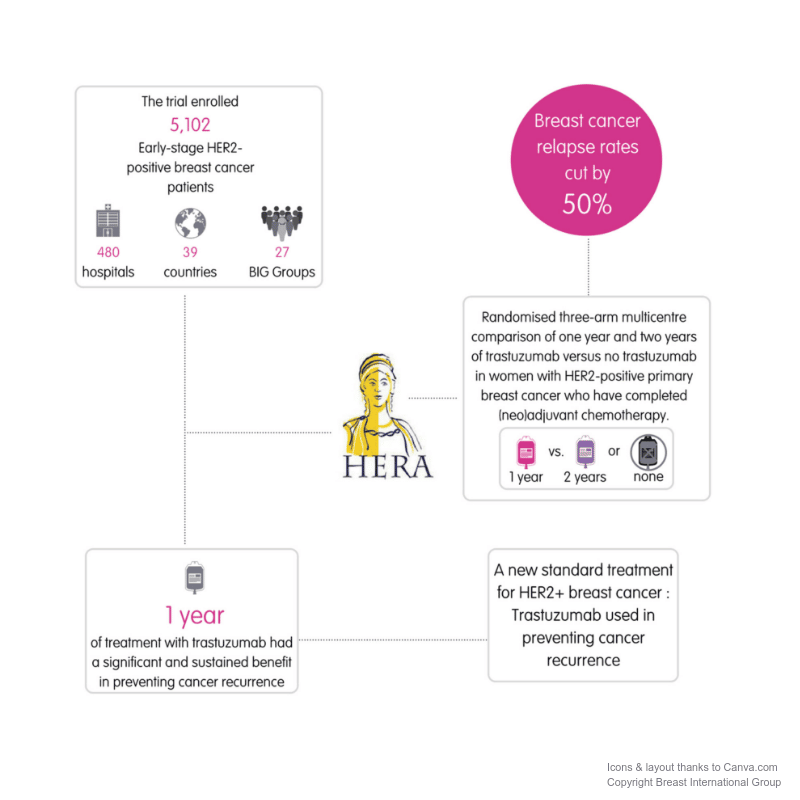
Researchers launched three clinical trials of trastuzumab in the mid-1990s for patients with HER2-positive metastatic breast cancer. By 1998, the results of the phase 3 clinical trials showed that breast cancer in patients treated with trastuzumab and chemotherapy grew at a slower rate than in patients treated with chemotherapy alone. Subsequent clinical trials also showed positive outcomes among women with early-stage HER2-positive breast cancer.
On November 16, 2006, the US Food and Drug Administration granted approval to trastuzumab used with chemotherapy as an adjuvant treatment for women with HER2-positive breast cancer. The drug has improved survival rates for women with stage 1 to 3 HER2-positive breast cancer by more than 30 percent.
HER2 protein is expressed at high levels in several other cancers besides breast cancer, and in 2010, FDA approved the use of trastuzumab in combination with the chemotherapy drug cisplatin and a type of cancer drug called a fluoropyrimidine to treat some patients with HER2-positive gastric or gastroesophageal junction cancers.
Read Also: Can You Live With Stage 4 Breast Cancer
The Need For New Treatment Options For Her2
Approximately 15-20% of all breast cancers test positive for human epidermal growth factor receptor 2 , a protein that promotes cancer growth. The research team at Moffitt developed a groundbreaking vaccine that helps the bodys immune system recognize the HER2 protein on breast cancer cells so that it can target and destroy those cells. This personalized vaccine is created from certain immune cells called dendritic cells, which are harvested from the patient.
In addition to this promising vaccine, the treatment options available to patients who are diagnosed with HER2-positive breast cancer recently expanded with the introduction of drugs that target the HER2 protein. However, because HER2-positive breast cancer can become resistant to the beneficial effects of these drugs over time, there is a need for additional treatment options.
New Drug Combination Recommended By Nice For Women With Advanced Her2+ Breast Cancer Following Royal Marsden Trial
The National Institute for Health and Care Excellence has recommended tucatinib, a type of targeted treatment, in combination with trastuzumab, another targeted treatment, and capecitabine, a chemotherapy, for previously treated patients with HER2-positive locally advanced or metastatic breast cancer.
This is the only clinically proven targeted drug combination that can extend overall survival in this patient group, as demonstrated by the HER2CLIMB study, a global, randomised, double-blind, phase II trial, which was supported by Royal Marsden researchers.
The study revealed that tucatinib in combination reduced patients risk of death by just over a third , and of disease progression or death by 46%, compared with trastuzumab and capecitabine alone. Overall survival was prolonged by 4.5 months compared to the control group. The study also revealed that, for patients with brain metastases, this combination reduced disease progression and risk of death by more than half and extended overall survival by 6 months.
NICEs recommendation follows the Scottish Medicines Consortium acceptance of this treatment for use in NHS Scotland in January. Around 400 patients are expected to benefit from the drug annually in England and Wales.
Dr Alicia Okines, Consultant Medical Oncologist at The Royal Marsden NHS Foundation Trust and team leader for the Breast Cancer Systemic Therapy Trials team at the Institute of Cancer Research, London said:
The HER2CLIMB study was funded by Seagen.
You May Like: Does Breast Cancer Cause High White Blood Cell Count
New Therapies For Her2
Over the past 18 months, the FDA has approved 4 new regimens for HER2-positive metastatic disease.
In an interview with Pharmacy Times, Kelly Gaertner, PharmD, an oncology clinical pharmacy specialist with Allegheny Health Network, said that a slew of new treatments for human epidermal growth factor receptor 2 -positive metastatic breast cancer offer exciting new possibilities for this patient population, although questions remain about optimal sequencing.
Gaertner will be presenting on the topic on Friday at the Hematology/Oncology Pharmacy Associations virtual 2021 conference.
Prior to December 2019, Gaertner said the standard first-line therapy for HER2-positive metastatic breast cancer was trastuzumab plus pertuzumab and a taxane. Second-line standard therapy involved ado trastuzumab emtansine. Third and subsequent lines of therapy had no standard regimen, however.
Over the past 18 months, the FDA has approved 4 new regimens for HER2-positive metastatic disease, including trastuzumab deruxtecan neratinib plus capecitabine tucatinib, capecitabine, and trastuzumab and the monoclonal antibody margetuximab. Although questions still remain about the optimal sequencing for these treatments in third and subsequent lines of therapy, Gaertner said the approvals are exciting developments.
Related Content:
New Treatment Approved For Her2
Breast cancer is the most common form of cancer in the U.S. According to the National Cancer Institute , approximately 15 percent of patients with breast cancer have tumors that are HER2-positive.
This summer, the U.S. Food and Drug Administration approved a new breast cancer treatment that aims to reduce the risk of the cancer returning. The drug, neratinib, is intended for patients with early-stage, HER2-positive breast cancer.
NCI estimates approximately 252,710 women will be diagnosed with breast cancer this year, and 40,610 will die of the disease. This approval is a major step forward in helping to improve survival rates in some breast cancer patients.
National Cancer Institute Food and Drug Administration
Image credit:
Don’t Miss: What Are The Side Effects Of Breast Cancer Treatment
Expanding Access To A Powerful Drug
Trastuzumab was the first targeted therapy developed to treat HER2-positive patients. HER2-positive, which accounts for 15% to 20% of all breast cancers, means there is a high level of the HER2 protein on the surface of cancer cells, driving tumor growth. Trastuzumab is a lifesaving drug that works by binding to HER2 and blocking tumor growth.
Unfortunately, many patients who initially respond to trastuzumab eventually develop resistance to the drug. In recent years, scientists have developed new drugs that target HER2 cancers more effectively. One of these drugs is T-DXd, which was originally approved by the U.S. Food and Drug Administration in 2019 for treating patients with HER2-positive, metastatic breast cancer who have stopped responding to other HER2 drugs. That approval was based on a study also in NEJM Dr. Modi was the first author.
Trastuzumab directly blocks growth signals sent out by HER2, but T-DXd works in a different way. With this drug, the trastuzumab is attached to a payload of chemotherapy, which it delivers to the site of the tumor. Because T-DXd is so much more powerful than standard trastuzumab, Dr. Modi and her colleagues hypothesized that it could be effective against tumors with low levels of HER2. More than 60% of tumors that traditionally have been labeled HER2-negative actually have some HER2 the group that was considered HER2-low in this study.
What Are The Goals Of Treatment
The goals of treatment depend on the stage of breast cancer you have at diagnosis. For those with stage 0 to 3 breast cancer, the goal of treatment is to cure the cancer and prevent future recurrence.
Stage 4 breast cancer means the cancer has spread beyond the breast and local lymph nodes. At this stage, the goal of treatment is to control the growth of the cancer and prevent any organ damage or pain.
Unfortunately, stage 4 breast cancer cannot be cured. But with the advent of new and innovative drugs, its possible to stay in a period of stable disease for long periods of time.
You May Like: Where Do You Check For Breast Cancer
Review Of The Tulip Trial
Overall responses were about 28% with the new molecule vs 30% with physicians choice, and target lesions responded 70% vs 58%. The clinical benefit ratesthis combination of , , and stable diseasewere 38.5% vs 32%.A hint at more activity, patients maintained their responses longer, and PFS was likely different: Based on that, looking positive for an efficacy signal.The other side of the coin, , is the toxicity , particularly ocular toxicity. What do you think about that and how were going to roll that into our practices? Apart from that, its not different from the physicians choice chemotherapy in terms of toxicity. There were few grade 3 or greater toxicities in terms of meaningful numbers, other than the ocular toxicity, which is a special interest here. Thats 30% of folks with events in the physicians choice arm vs 78% with the new molecule for ocular toxicity, and the grade 3 rate was 21%. That is a lot of serious dry eye, and the requirement that were going to need ocular oncologist involved in this. Pneumonitis is generally low grade, and grade 3 events were around 2.5%, not in the ballpark of where we see deruxtecan.In a polling question, audience members indicated that the DESTINY-Breast03 trial was the most relevant to their practice .