The Flip Side Of The Coin: Immune
The price of improving patients outcomes with the addition of immunotherapy is the risk of irAEs beyond the toxicities of traditional chemotherapy. The most common irAEs observed in KEYNOTE-522 were infusion reactions , thyroid impairment , skin toxicities , pneumonitis , hypophysitis , colitis and hepatitis in combined neoadjuvant and adjuvant phases. Importantly, some of these are expected to be irreversible, permanently conditioning the quality of life of patients in this curable setting. Additionally, concern on the impact of immunotherapy on fertility exist, particularly since TNBC often occurs in pre-menopausal patients. In this regards, appropriate training of clinicians in the early identification and management of irAEs will be key for the mitigation of immunotherapy side effects. Concomitantly, these risks should be discussed with patients upfront, to provide a clear overview of the risks/benefits balance of adding immunotherapy to chemotherapy for the treatment of their tumor.
The Rise Of Immunotherapy For Tnbc
Despite lacking canonical targets for biologic treatment, TNBC is characterized by a relatively high tumor mutational burden compared to other subtypes of BC, a feature which has been linked with increased responsiveness to immunotherapy with immune-checkpoint inhibitors . Indeed, checkpoint inhibition with atezolizumab and with pembrolizumab has been approved for advanced-stage, PD-L1 positive TNBC based on the improvement in outcomes observed when combined with frontline chemotherapy,. Notably, evidence suggest a superior efficacy of ICIs in TNBC when administered early in the disease course, possibly due to the progression of immune escape mechanisms during the advancement of disease,. From this perspective, there was strong rationale for ICI administration to the earliest possible time in the disease course, namely before surgical resection. Results from several randomized trials designed with this purpose are now available, igniting a rapid change of practice in early TNBC.
Table 1 Features and outcomes of the main randomized chemo-immunotherapy trials in early-stage triple negative breast cancer.
Asco Guideline Rapid Update Addresses The Use Of Neoadjuvant Pembrolizumab In High
A rapid update to the ASCO Guideline on neoadjuvant therapy for breast cancer adds a recommendation on the use of pembrolizumab in patients with high-risk early-stage triple-negative breast cancer.1 The update follows a recent analysis from the randomized phase III KEYNOTE-522 trial that showed a significant event-free survival benefit with the addition of pembrolizumab to standard neoadjuvant chemotherapy followed by adjuvant pembrolizumab after surgery in patients with high-risk triple-negative breast cancer.2
The results from this study led to the U.S. Food and Drug Administration approval of pembrolizumab in this setting.3 This is the first FDA approval of an immunotherapy agent in early-stage breast cancer, noted Larissa A. Korde, MD, MPH, FASCO, of the National Cancer Institute, and Guideline Co-Chair.
Larissa A. Korde, MD, MPH, FASCO
Evidence for Guideline Update
KEYNOTE-522 was a randomized, multicenter, double-blind, placebo-controlled trial designed to compare neoadjuvant carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide plus either pembrolizumab or placebo , followed by pembrolizumab or placebo administered every 3 weeks for up to nine cycles after surgery, in patients with previously untreated stage II/III triple-negative breast cancer.2
Dawn L. Hershman, MD, MS, FASCO
Ongoing Questions
REFERENCES
Originally published in ASCO Daily NewsASCO Daily News, April 20, 2022. All rights reserved.
Also Check: What Is Estrogen Breast Cancer
Biomarkers For Immunotherapy In Tnbc
Despite this deeper understanding of TNBC immunobiology, further development of suitable biomarkers for immunotherapy response and benefit in patients with early-stage TNBC remains an area of significant need. The standardization of biomarker assay implementation and interpretation methodologies before introducing novel biomarkers into the clinical setting will be required. Strategies to identify and target key immune suppression regulators within the tumor microenvironment that could contribute to immunotherapy resistance or predict benefit to checkpoint inhibition and optimize tumor immunogenicity will achieve a more personalized therapeutic approach and balance the risks of immunotherapy-related toxicity.
Research Progress And Future Questions
After the 2020 approval of the combination of pembrolizumab and chemotherapy for advanced triple-negative breast cancer, FDA approved the combination therapy for people with early-stage disease in 2021.
That approval was based on results from a different trial, KEYNOTE-522. In that study, patients with high-risk, early-stage triple-negative breast cancer benefited from pembrolizumab given with chemotherapy before surgery, and then continued as a single agent as an additional, or adjuvant, treatment after surgery.
This is an exciting time for research on triple-negative breast cancer, said Dr. Lee. We have now seen a benefit from an immune checkpoint inhibitor and chemotherapy in a subgroup of patients in both the advanced and early stages of the disease.
Dr. Lee cautioned, however, that more than half of all patients with triple-negative breast cancer have PD-L1 combined positive scores of less than 10, so more work is needed to find effective treatments for these patients.
In his editorial, Dr. Pivot noted that people diagnosed with triple-negative breast cancer are not a homogeneous group. Future studies, he added, will try to identify which individuals are more or less likely to benefit from pembrolizumab.
You May Like: Can Sleeping On Your Breast Cause Cancer
Pembrolizumab Plus Chemotherapy In Triple
- ContactAffiliationsDepartment of Breast Medical Oncology, Breast Oncology Center, The Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan
- Yukinori OzakiAffiliationsDepartment of Breast Medical Oncology, Breast Oncology Center, The Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan
- Fumikata HaraAffiliationsDepartment of Breast Medical Oncology, Breast Oncology Center, The Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan
- Shigehisa KitanoAffiliationsDivision of Cancer Immunotherapy Development, Advanced Medical Development Center, The Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan
- Toshimi TakanoAffiliationsDepartment of Breast Medical Oncology, Breast Oncology Center, The Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan
Lancet.
Ann Oncol.
N Engl J Med.
Lancet Oncol.
Nice Draft Guidance Does Not Recommend Pembrolizumab Plus Chemotherapy For Triple Negative Breast Cancer
Draft guidance published today by NICE for public consultation does not recommend pembrolizumab plus chemotherapy for treating triple negative breast cancer that has spread to other parts of the body.
08 March 2022
The evidence showed that pembrolizumab plus chemotherapy is more effective than paclitaxel or nab-paclitaxel, although the long-term benefit is uncertain. In addition, there was no trial data directly comparing pembrolizumab plus chemotherapy with atezolizumab plus chemotherapy, another targeted treatment which NICE already recommends.
Therefore, the cost-effectiveness estimates are higher than NICE normally considers an acceptable use of NHS resources.
The committee would like to see more information from the company about the comparison between pembrolizumab plus chemotherapy with atezolizumab plus chemotherapy.
Helen Knight, programme director in the NICE Centre for Health Technology Evaluation, said:I know that todays announcement will be disappointing for people with this type of breast cancer, as well as for their families and carers. Advanced triple negative breast cancer has a significant negative impact on quality of life. It can be more aggressive than other types of breast cancer and accounts for a quarter of all deaths from breast cancer despite accounting for only 1 in 5 cases.
Around 600 people in England with advanced triple negative breast cancer would have been eligible for treatment with the pembrolizumab combination.
Don’t Miss: What Does Stage 2b Breast Cancer Mean
Nice Allows Immunotherapy Treatment For Small Group Of Patients With Advanced Triple Negative Breast Cancer
The National Institute for Health and Care Excellence has now ruled that pembrolizumab plus chemotherapy should be available as a treatment option for a limited group of patients with advanced triple negative breast cancer.1
In March 2022 NICE published draft guidance that said the immunotherapy treatment was not a cost effective use of resources.2
Pembrolizumab, which is given by injection every three weeks, is a type of immunotherapy that works by blocking the protein PD-L1 which is produced in larger amounts on cancerous cells than normal cells. By blocking PD-L1 it helps the persons own immune cells to attack the cancer.
Following consultation, the drugs manufacturer Merck Sharp and Dohme proposed that the pembrolizumab combination should be restricted to people whose tumours express PD-L1 with a combined positive score of 10 or more and immune cell staining of less than 1%. This is narrower than the marketing authorisation.
NICE already recommends another immunotherapy treatment, atezolizumab plus chemotherapy, for people with locally advanced or metastatic triple negative breast cancer with an IC equal to or greater than 1%.3 This means that some people that cant have atezolizumab combination could be eligible for pembrolizumab combination and NICEs independent appraisal committee decided that it therefore fulfils an unmet need.
Identifying Responders To Immunotherapy: Current Status And Future Perspectives
KEYNOTE-522 results prompted a rapid change in clinical practice, leading to the FDA approval of the first immunotherapy agent for early-stage TNBC. This landmark achievement, however, has raised a multitude of scientific questions, requiring a new set of prospective clinical trials.
Besides baseline biomarkers, one established dynamic biomarker, namely the achievement of pCR after neoadjuvant treatment, showed a critical value in KEYNOTE-522. Indeed, a major absolute benefit in terms of EFS was observed among patients not achieving pCR, with a 10% improvement in 3-year EFS for patients receiving pembrolizumab, whereas only a 2% difference was observed in those patients achieving pCR. This finding – together with the results of GeparNuevo showing survival outcomes similar to KEYNOTE-522 with immunotherapy administered only before surgerysupport the experimental testing of strategies to de-escalate adjuvant immunotherapy in patients achieving pCR with chemo-immunotherapy. Nonetheless, until prospective evidence is available, current standards of care should include the adjuvant administration of pembrolizumab to all patients receiving it in the neoadjuvant setting without experiencing concerning irAEs. Moreover, when comparing EFS curves from patients achieving pCR in the two arms, it is important to stress the fact that the addition of pembrolizumab led to more patients achieving pCR, ultimately enriching the population of patients achieving a favorable EFS.
Recommended Reading: What Are The Most Common Signs Of Breast Cancer
Pembrolizumab Granted 4 Approvals In Japan For High
Japans Ministry of Health, Labor and Welfare approved pembrolizumab for use in 4 indications, including high-risk, early-stage triple-negative breast cancer, stage IIB or IIC melanoma, adjuvant renal cell carcinoma, and recurrent/metastatic cervical cancer.
Japans Ministry of Health, Labor and Welfare approved pembrolizumab both as a single agent and in combination with other drugs across several disease types, including in combination with neoadjuvant chemotherapy in hormone receptornegative, HER2-negative triple-negative breast cancer at a high risk of recurrence in adjuvant renal cell carcinoma at a high risk of recurrence post-nephrectomy or nephrectomy and metastatic lesion resection in combination with chemotherapy plus or minus bevacizumab for advanced or recurrent cervical cancer with no previous chemotherapy use and adjuvant stage IIB or IIC fully resected melanoma, according to a press release from Merck.1
New Treatments Needed For Advanced Triple
Triple-negative breast cancer tends to be more aggressive, harder to treat, and more likely to recur than other forms of the disease, such as hormone receptorpositive or HER2-positive breast cancers.
Conventional chemotherapy drugs have not been effective against triple-negative breast cancer, and new treatment options are needed, said Jung-Min Lee, M.D., of the Womens Malignancies Branch in NCIs Center for Cancer Research.
In the KEYNOTE-355 trial, 847 patients with advanced triple-negative breast cancer were randomly assigned to receive chemotherapy plus placebo or chemotherapy plus pembrolizumab.
The study assessed the amount of time before the disease worsened and overall survival in all patients, in those with PD-L1 combined positive scores of 1 or more, and in those with combined positive scores of 10 or more. The trial was funded by Merck, the manufacturer of pembrolizumab.
The PD-L1 combined positive score is essentially a measure of the extent to which cells in a tumor produce PD-L1, the immune checkpoint protein that pembrolizumab targets. By blocking immune checkpoints, pembrolizumab and other immune checkpoint inhibitors unleash the immune system against cancer cells.
The incidence of treatment-related side effects, including serious side effects, was similar between the two groups of patients in the study.
Read Also: Encouraging Words For Breast Cancer Patients
Pembrolizumab In Treatment Of Triple
Breast cancer. 3d illustration
Pembrolizumab in combination with chemotherapy is already FDA-approved for the treatment of patients with triple-negative breast cancer in the setting of early or metastatic diseases.
Here we approach the main studies that led to these approvals. Annually in Brazil the breast cancer represents approximately 28% of new cases of cancer being the second type of neoplasm more incident among women. The diseases are considered heterogenous and its molecular subtype differ in genomic complexity, major genetic alterations, and clinical prognosis.
The group of patients with the disease that expresses hormone receptors is the most prevalent represents approximately 70% of cases. The group of patients HER2+ is the group referred triple-negative breast cancer , represent respectively about 20% and 15% of patients with this type of cancer.
In that regard, triple negative breast cancer differs from other types of breast cancer in that it grows and spreads faster, in addition to having limited treatment options and worse prognosis.
Pembrolizumab is a highly selective, humanized, PD-1-targeted monoclonal antibody. This drug has demonstrated robust antitumor activity and a favorable safety profile in several types of tumors, which has led to regulatory approval by ANVISA in at least 20 different indications. It has been investigated alone and in combination with other antitumors therapies.
1. Banerji S Nature.2012Jun 20 486:405-9.
Pembrolizumab Monotherapy In Metastatic Triple
- ContactAffiliationsDivision of Medical Oncology, Princess Margaret Cancer Centre and the University of Toronto, Toronto, ON M5G 2M9, Canada
- David W CesconAffiliationsDivision of Medical Oncology, Princess Margaret Cancer Centre and the University of Toronto, Toronto, ON M5G 2M9, Canada
Clin Cancer Res.
Ann Oncol.
Don’t Miss: What Are The First Signs Of Breast Cancer
Pembrolizumab Plus Chemotherapy Extends Survival In Triple
Disclosures: We were unable to process your request. Please try again later. If you continue to have this issue please contact .
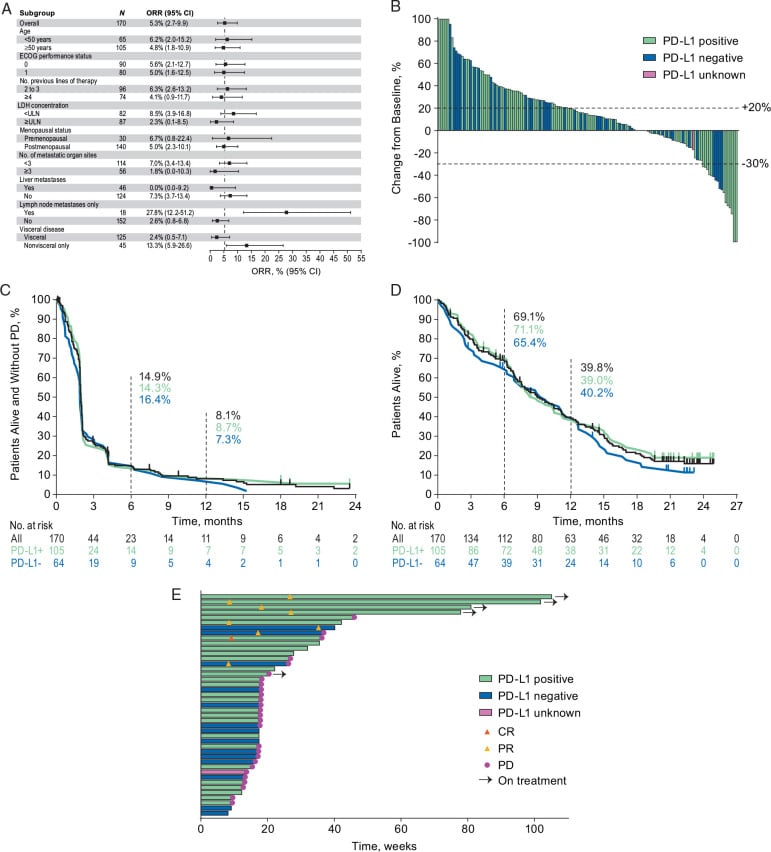
- Researchers report nearly 7-month increase in median OS among patients with a PD-L1 CPS of 10 or higher.
- Safety consistent with previous analyses.
- Immunotherapy class marks the continued improvement of breast cancer treatment.
The addition of pembrolizumab to first-line chemotherapy significantly prolonged OS compared with chemotherapy alone among a subgroup of patients with inoperable or metastatic triple-negative breast cancer, according to study results.
Javier Cortes
Findings of the phase 3 KEYNOTE-355 trial, published in The New England Journal of Medicine, showed the OS benefit specifically among patients with tumors that expressed PD-L1 with a combined positive score of 10 or higher.
Rationale and methods
As Healio previously reported, the KEYNOTE-355 trial included 847 patients with previously untreated, locally recurrent inoperable or metastatic disease whose tumors expressed PD-L1.
In the final OS analysis, Cortes and colleagues reported outcomes in subgroups of patients with PD-L1 CPS of 10 or higher , a PD-L1 CPS of 1 or higher and in the intention-to-treat population.
Median follow-up was 44.1 months.
Key findings
No new safety signals were reported.
Implications
References:
Dr Oshaughnessy On Pembrolizumab In Triple
Joyce A. OShaughnessy, MD, co-chair, Breast Cancer Research, chair, Breast Cancer Prevention Research, Baylor-Sammons Cancer Center, The US Oncology Network member, Scientific Advisory Board, US Oncology Research Network, discusses the sequencing of pembrolizumab with chemotherapy in patients with early-stage triple-negative breast cancer .
Pembrolizumab is currently indicated in patients with stage II or III TNBC and no history of serious autoimmune disorders who will be receiving preoperative chemotherapy, OShaughnessy says. For instance, the regimen from the phase 3 KEYNOTE-522 trial is preoperative paclitaxel, carboplatin, AC , and pembrolizumab, followed by surgery and radiation with concurrent pembrolizumab, for a total of 6 months of postoperative pembrolizumab therapy, OShaughnessy explains.
For patients with BRCA1 and BRCA2 wild-type disease, several meta-analyses and clinical trials have demonstrated improved survival with 6 months of oral capecitabine without pembrolizumab, OShaughnessy notes. Additionally, a regimen of 6 months of capecitabine plus pembrolizumab is being investigated in patients with residual disease, OShaughnessy says. Although level 1 evidence has yet to be determined for the combination compared with either agent alone, the safety data for the combination looks promising, OShaughnessy concludes.
Also Check: What Is Her Negative Breast Cancer
Ivermectin And Pembrolizumab For The Treatment Of Metastatic Triple Negative Breast Cancer
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.Know the risks and potential benefits of clinical studies and talk to your health care provider before participating. Read our disclaimer for details. |
| Recruitment Status : Not yet recruitingFirst Posted : April 8, 2022Last Update Posted : July 27, 2022 |
| Anatomic Stage IV Breast Cancer AJCC v8Metastatic Triple-Negative Breast Carcinoma | Drug: IvermectinBiological: PembrolizumabOther: Quality-of-Life Assessment | Phase 2 |
PRIMARY OBJECTIVES:
I. To determine the efficacy of ivermectin in combination with pembrolizumab in metastatic triple negative breast cancer using objective response rate .
II. To assess the safety and tolerability of ivermectin in combination with pembrolizumab using National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 in three doses.
SECONDARY OBJECTIVES:
I. To determine the optimal biological dose of ivermectin when administered with pembrolizumab.
II. To evaluate the following efficacy outcomes: progression free survival , overall survival , duration of response , clinical benefit rate .
EXPLORATORY OBJECTIVES:
OUTLINE: This is a dose-escalation study of ivermectin.
Research Agenda For The Next Decade
The introduction of immunotherapy marks a revolution in the treatment of early-stage TNBC. KEYNOTE-522 has shown that, by unleashing anti-cancer immune responses through ICIs, long-term benefits can be obtained for the treatment of this aggressive BC subtype. However, it represents a starting point rather than a finish line, and additional efforts will be required precisely implement immunotherapy for the treatment of TNBC .
Fig. 1: Next decade research agenda for neo immunotherapy in TNBC.
Abbreviations: IO, immunotherapy, TNBC, triple negative breast cancer TMB, tumor mutational burden ADC, antibody-drug conjugate ER, estrogen receptor CD, cluster of differentiation TILs, tumor infiltrating lymphocytes PD-L1, Programmed death-ligand 1 HLA, human leukocyte antigen PD-1, Programmed cell death protein 1 A, adenosine T, thymine C, cytosine G, guanine BRCA, BReast CAncer gene EFS, event-freee survival RD, residual disease me1, mono-methylated form BC, breast cancer. Created with biorender.com.
Don’t Miss: Stage Iv Triple Positive Breast Cancer