Combination Her2 Directed Therapy
The combination of lapatinib and trastuzumab has demonstrated synergy in preclinical models and has recently been shown to improve PFS in patients with MBC that had progressed on trastuzumab . In a phase III trial, 296 HER2-positive patients whose disease had progressed on trastuzumab-containing regimens were randomized to receive lapatinib alone or lapatinib with trastuzumab. The median PFS was 12.0 weeks in the lapatinib plus trastuzumab arm and 8.1 weeks in the lapatinib arm . The combination of trastuzumab and lapatinib was well tolerated, with fewer serious adverse events than would be expected with a chemotherapy containing regimen . In an updated analysis, the median OS was 14 months for the lapatinib plus trastuzumab arm, compared with 9.5 months for the lapatinib arm .
Early Stage Breast Cancer
After its approval in the metastatic setting, trastuzumab was evaluated in the adjuvant setting in several large prospective randomized trials that enrolled over 13,000 patients in total. The addition of trastuzumab to chemotherapy led to a significant improvement in DFS and OS . As expected, the benefit of trastuzumab is limited to HER2-positive disease . The first regimen evaluated with trastuzumab was doxorubicin, cyclophosphamide followed by paclitaxel, trastuzumab . While effective, this comes at the cost of a 4.1% risk of grade 3/4 heart failure which is attributable to the use of an anthracycline with trastuzumab . A non-anthracycline-based regimen was also evaluated in a trial that enrolled 3,222 women with HER2-amplified early breast cancer and randomly assigned them to receive chemotherapy alone , anthracycline-based chemotherapy plus trastuzumab , or a non-anthracycline based chemotherapy plus trastuzumab . Efficacy analysis at 65 months demonstrated that women receiving either trastuzumab-containing regimen had significantly improved DFS and OS compared to women receiving ACâT. Additionally, there was no statistically significant difference in DFS between the TCH and ACâTH arms. However, there were fivefold greater congestive heart failure events with ACâTH than with TCH . These data support the use of trastuzumab with a non-anthracycline based chemotherapy regimen in the adjuvant treatment of early breast cancer.
Can Fish Test For Her2
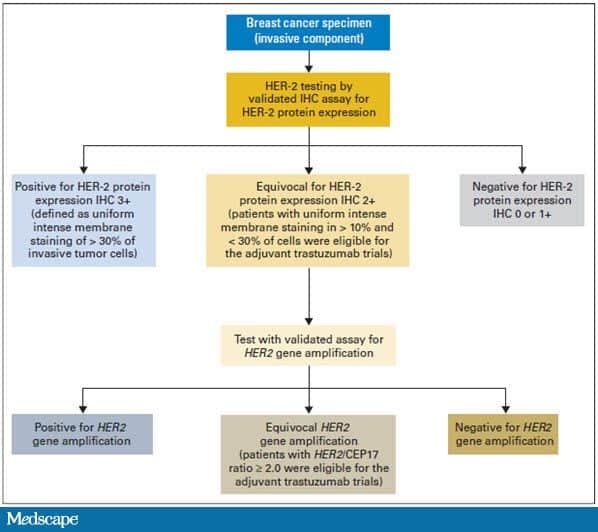
This means that the HER2 status needs to be tested with FISH to clarify the result . Triple-negative breast tumors dont have too much HER2 and also dont have estrogen or progesterone receptors. They are HER2-, ER-, and PR-negative. Hormone therapy and drugs that target HER2 are not helpful in treating these cancers.
Also Check: Ultra Sound For Breast Cancer
What Is Her2 In Breast Cancer
HER2 is a growth-promoting protein on the outside of all breast cells . Breast cancer cells with higher than normal levels of HER2 are called HER2-positive. These cancers tend to grow and spread faster than other breast cancers, but are much more likely to respond to treatment with drugs that target the HER2 protein.
What Are The Symptoms Of Her2
Its not possible to self-determine whether you have HER2-positive breast cancer. If your doctor suspects cancer, further testing will reveal whether you are HER2-positive.
Overall, its important to see your doctor right away if you notice any of the following symptoms:
- any new or changing lumps in your breast or armpit areas
- clear, colored, or bloody nipple discharge
- unexplained pain in your breasts
- changes in your nipples or breast skin, such as dimpling, reddening, or scaliness
- nipples that turn inward
Hormone treatments may be an option for cancer thats also HR-positive.
Recommended Reading: What Is The Survival Rate For Stage 3 Breast Cancer
Different Drugs Same Target
Breast cancers that are HER2-positive tend to be aggressive, with the excess HER2 protein on tumor cells fueling the cancers growth. In the late 1990s, trastuzumab was among the first targeted cancer therapies to be approved by FDA, after trials showed it could improve survival in women with metastatic HER2-positive breast cancer.
Over time, other HER2-targeted therapies emerged, some with alternative mechanisms for disrupting HER2 activity in cancer cells. Drugs like trastuzumab and pertuzumab are monoclonal antibodies that bind to the HER2 protein above the cancer cells surface, preventing it from acting or enlisting the immune system to help destroy cells that produce it.
Tucatinib, on the other hand, is a member of a class of drugs known as tyrosine kinase inhibitors . These drugs work by binding to the part of the HER2 protein that is inside the cell and preventing it from sending signals that promote cell growth. Other HER2-targeted TKIs include neratinib and lapatinib .
Some TKIs have multiple targets. But, compared with other HER2-targeted drugs, tucatinib appears to be relatively selective for HER2that is, its less likely to bind to related proteins, explained Stanley Lipkowitz, M.D., Ph.D., chief of the Womens Malignancies Branch in NCIs Center for Cancer Research. That selectivity limits the risk of side effects seen with other HER2-targeted TKIs that inhibit other targets, Dr. Lipkowitz said.
Is Her2 A Tumor
Approximately 20% of women are diagnosed with tumors deemed HER2-positive, those that have an amplification of the HER2 gene. HER2-positive tumors are a relatively aggressive form of breast cancer, but since its first approval in 1998, trastuzumab has improved the survival for these patients. The drug is most frequently used in the adjuvant setting after surgical removal of the tumor.
Also Check: What Is The Worst Form Of Breast Cancer
Your Treatment Is Unique
HER2-positive breast cancer is different from other breast cancer types, so your treatment wonât necessarily be the same as someone else who has a different form of breast cancer. It may also be different than another HER2-positive patientâs therapy.
Each cancer is unique, so doctors try to develop the treatment course thatâs best for you. Things to consider include the size of your tumor, whether the cancer has metastasized , or your overall risk of recurrence.
Are A Woman Who Could Become Pregnant Or May Be Pregnant
HERCEPTIN HYLECTA may result in the death of an unborn baby or birth defects. Contraception should be used while receiving HERCEPTIN HYLECTA and for 7 months after your last dose of HERCEPTIN HYLECTA. If you are or become pregnant while receiving HERCEPTIN HYLECTA or within 7 months after your last dose of HERCEPTIN HYLECTA, you are encouraged to report HERCEPTIN HYLECTA exposure to Genentech at 18888352555.
Read Also: Does Breast Cancer Always Come Back
Other Treatments For Her2 Positive Breast Cancer
In addition to HER2-targeted therapies, there are also several standard chemotherapy drugs that are used to treat HER2 positive breast cancer. While these medications do not target the HER2 protein specifically, many patients have experienced positive results through the use of these medications.
Additionally, treatment for HER2 positive breast cancer is advancing every day. Several new medications that also target the HER2 protein are being tested in clinical trials. Moffitt has been designated as a Comprehensive Cancer Center by the National Cancer Institute, an achievement that speaks directly to our dedication to advancing cancer research and treatment. Through our robust clinical trials program, eligible patients have the opportunity to benefit from the latest breakthroughs in treatment before they are made available elsewhere.
To speak with a Moffitt physician about your HER2 positive breast cancer treatment options, request a consultation by completing a new patient registration form online or calling . We do not require referrals.
Targeted Therapy For Women With Brca Gene Mutations
Olaparib and talazoparib are drugs known as PARP inhibitors. PARP proteins normally help repair damaged DNA inside cells. The BRCA genes also help repair DNA , but mutations in one of those genes can stop this from happening. PARP inhibitors work by blocking the PARP proteins. Because tumor cells with a mutated BRCA gene already have trouble repairing damaged DNA, blocking the PARP proteins often leads to the death of these cells. These drugs are pills and are taken once or twice a day. They can be used in different ways to treat breast cancer.
-
Olaparib can be given to women with a BRCA mutation with early-stage HER2-negative breast cancer after surgery who have been treated with chemotherapy and are at high risk of the cancer recurring. It is typically given for one year. When given in this way, it can help some women live longer.
-
Olaparib and talazoparib can be used to treat advanced or metastatic, HER2-negative breast cancer in women with a BRCA mutation who have already had chemotherapy. If the cancer is hormone receptor-positive, olaparib can also be used in women who have already received hormone therapy.
Side effects can include nausea, vomiting, diarrhea, fatigue, loss of appetite, taste changes, low red blood cell counts , low platelet counts, and low white blood cell counts. Rarely, some people treated with a PARP inhibitor have developed a blood cancer, such as myelodysplastic syndrome or acute myeloid leukemia .
Recommended Reading: How Do You Check For Breast Cancer
Whats The Difference Between Her2
HER2 proteins can indicate whether breast cancer cells are likely to divide and replicate. HER2-negative breast cancer is more common and means that cancer cells are not producing a lot of HER2.
HER2-positive breast cancer, on the other hand, means that the cells are producing a large number of these hormone receptors, indicating a more aggressive cancer.
- having a history of receiving radiation therapy in your chest area
- smoking or using other tobacco products
Also, while having a family history of breast cancer generally increases your personal risk of breast cancer development, HER2-positive breast cancer is not hereditary.
Effects On Different Races And Ethnicities
In general, young women and Black women have a higher chance of being diagnosed with triple-negative breast cancer than older women and women of other races or ethnicities. The American Cancer Societyâs Breast Cancer Facts & Figures 2019-2020 reports that of all breast cancers diagnosed in Black women, about 21% are triple-negative. In comparison, only 10% of breast cancer diagnosed in non-Hispanic white women and Asian/Pacific Islanders is triple-negative, and only 12% of breast cancer diagnosed in American Indian/Alaska Native women and Hispanic women is triple-negative.
Though Black women have the highest chance of being diagnosed with triple-negative disease compared to women of other races, the rate of triple-negative diagnosis in Black women is still lower than diagnosis of other breast cancers. Like women from all racial and ethnic groups, Black women are most often diagnosed with hormone receptor-positive breast cancer. Of breast cancers diagnosed in Black women, 61% are hormone receptor-positive.
Recommended Reading: How To Prevent Breast Cancer Naturally
Expanding Access To A Powerful Drug
Trastuzumab was the first targeted therapy developed to treat HER2-positive patients. HER2-positive, which accounts for 15% to 20% of all breast cancers, means there is a high level of the HER2 protein on the surface of cancer cells, driving tumor growth. Trastuzumab is a lifesaving drug that works by binding to HER2 and blocking tumor growth.
Unfortunately, many patients who initially respond to trastuzumab eventually develop resistance to the drug. In recent years, scientists have developed new drugs that target HER2 cancers more effectively. One of these drugs is T-DXd, which was originally approved by the U.S. Food and Drug Administration in 2019 for treating patients with HER2-positive, metastatic breast cancer who have stopped responding to other HER2 drugs. That approval was based on a study also in NEJM Dr. Modi was the first author.
Trastuzumab directly blocks growth signals sent out by HER2, but T-DXd works in a different way. With this drug, the trastuzumab is attached to a payload of chemotherapy, which it delivers to the site of the tumor. Because T-DXd is so much more powerful than standard trastuzumab, Dr. Modi and her colleagues hypothesized that it could be effective against tumors with low levels of HER2. More than 60% of tumors that traditionally have been labeled HER2-negative actually have some HER2 the group that was considered HER2-low in this study.
Choosing Therapy Based On Recent Data
Mahtani: I share your on that forest plot where you dont see a differential benefit in ER positive vs ER-negative, HER2-positive patients. In the earlier setting, when we used the CLEOPATRA regimen , many of us dropped the taxane and started hormonal therapy as a maintenance with the backbone.4 There are ongoing trials looking at the benefit of CDK4/6 inhibitors on top of that. The PATINA study is looking at that approach, plus or minus a CDK4/6 inhibitor. Theres rationale in considering that approach, but in the setting of the data we looked at, Id feel comfortable giving a patient with ER-positive, HER2-positive disease trastuzumab deruxtecan, knowing that the ER expression will not differentially affect their benefit.Gadi: Interstitial lung disease is a toxicity that we dont have a lot of leeway with the label and guidance from the company are very clear. If you suspect ILD, the recommendation is to hold the drug if its grade 1. If its grade 2, 3, or 4, the recommendation is to discontinue the drug. Youll have a patient in front of you, and youll make the right choice, but for grade 1 toxicities you may or may not need to start steroids. Hold the drug, let it resolve, and revisit the issue. If the risk-benefit ratio is in favor of restarting, then restart the same dose or a lower dose, whichever the guidance is, based on what youre observing. Its not worth crossing that line with this drug.
Read Also: How Do Doctors Check For Breast Cancer
Causes And Risk Factors Of Her2 Positive Breast Cancer
There are several lifestyle, environmental and genetic risk factors for breast cancers, including HER2 positive breast cancer.
- HER2 breast cancer is not hereditary. It is caused by the excess production of HER2 protein.
- The risk of breast cancer in general increases if you have breast cancer cases in the family.
- Being overweight or obese increases the risk of developing breast cancer in post-menopausal women, including HER2 breast cancer.
- Living a sedentary lifestyle, smoking, and drinking alcohol can also increase an individuals risk of developing breast cancer.
- Other risk factors may include ageing, previous breast cancer diagnoses, having dense breasts , having radiation to the chest area before the age of 30, beginning menstruation before age 12, and having mutations of genes such as BRCA1, BRCA2as well as less common mutations in PTEN, PALB2, CHEK2, ATM, CDH1 and P53.
Pursuing The Causes For Fundamental Improvement
First, the economic factors.
Second, the standardized treatment.
In addition to economic factors, the clinicians treatment concept is bound to have some impact on the use of targeted drugs. Targeted therapy, as a safe and effective anti-tumor treatment, by virtue of its advantages, plays an increasingly important role in tumor treatment. It has become the choice for cancer treatment as important as surgery, radiotherapy and chemotherapy cancer treatment can be described as entering the period of targeted therapies.
Currently, many clinicians in first-tier cities have accepted the concept of targeted therapy. But relatively big gaps can still be found when comparing data on the proportion of targeted therapy in different provinces, or in different cities in a province. For example, in Guangdong province, statistical data on the rate of targeted therapy for HER2-positive breast cancer patients has revealed occurrences of 0% in some hospitals. Therefore, the advantages of targeted therapy and its importance in the overall treatment of breast cancer need to be emphasized, and the regular requirement of targeted therapy needs to be standardized to avoid any adverse effect on the patient survival as a result of poor treatment concept.
You May Like: When Does Breast Cancer Develop
Fda Approves New Treatment Option For Patients With Her2
- For Immediate Release:
This press release was updated to indicate that this application was approved four months prior to the FDA goal date.
Today, the U.S. Food and Drug Administration granted accelerated approval to Enhertu for the treatment of adults with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting. Enhertu is a human epidermal growth factor receptor 2 -directed antibody and topoisomerase inhibitor conjugate, meaning that the drug targets the changes in HER2 that help the cancer grow, divide and spread, and is linked to a topoisomerise inhibitor, which is a chemical compound that is toxic to cancer cells.
There have been many advances in the development of drugs for HER2-positive breast cancer since the introduction of Herceptin in 1998. The approval of Enhertu represents the newest treatment option for patients who have progressed on available HER2-directed therapies, said Richard Pazdur, M.D., director of the FDAs Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDAs Center for Drug Evaluation and Research. Drug development in the area of targeted therapies builds on our scientific understanding of malignant diseases not only in breast cancer, but in multiple other diseases.
The FDA granted the approval of Enhertu to Daiichi Sankyo.
A Number Of Clinical Studies Provided Evidence
Since the development and usage of trastuzumab, the international field of breast cancer study has carried out a number of large scale classic adjuvant therapy in clinical studies, including NSABP B-31, NCCTG9831, HERA, FinHer and BCIRG006, etc. , all having explored the efficacy, the treatment time and the best treatment modality using trastuzumab in HER2-positive breast cancer patients. The results of these studies have subsequently confirmed that the adoption of trastuzumab for HER2-positive breast cancer can reduce the risk of recurrence of early breast cancer by about 50% and lower the risk of death by about 30%.
N9831 and NSABP B-31 studies have compared the adjuvant treatment efficacy in HER2-positive breast cancer patients with AC-T program with or without trastuzumab, jointly analysing the patient overall survival and disease-free survival . The results showed that the DFS rate of patients with trastuzumab had significantly improved over the group of patients without trastuzumab treatment , the OS rate was also significantly increased . The cumulative incidences of distant metastasis in the trastuzumab usage group had an absolute reduction of 9.6%.
Read Also: Breast Cancer Education And Awareness